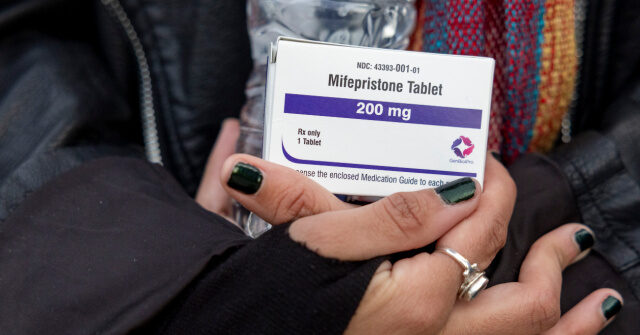
The U.S. Food and Drug Administration (FDA) approved a new generic version of the abortion pill on Tuesday, prompting outrage from conservatives and the pro-life movement.
The FDA’s approval of a generic form of mifepristone — the first drug used in a two-drug medication abortion regimen — comes as the agency, under the direction of the Department of Health and Human Services (HHS), is performing a safety review of the drug following a shocking study suggesting a much higher complication rate than previously reported.
The FDA specifically approved a generic form of the abortion pill produced by Evita Solutions LLC, a company that says its mission is to “normalize abortion” and make it “accessible to all.” In the FDA’s letter to Evita Solutions LLC, the agency said it concluded the drug is “bioequivalent and therapeutically equivalent” to the brand name mifepristone drug Mifeprex, which is made by Danco Laboratories, and has therefore met the requirements for approval.
Breitbart News reached out to the FDA for comment about the drug’s approval and asked whether the agency is still conducting a safety review of mifepristone.
HHS communications director Andrew Nixon told Breitbart News the FDA has “very limited discretion in deciding whether to approve a generic drug.”
“By law, the Secretary of Health and Human Services must approve an application if it demonstrates that the generic drug is identical to the brand-name drug,” Nixon said.
Continue Reading on Breitbart
This preview shows approximately 15% of the article. Read the full story on the publisher's website to support quality journalism.