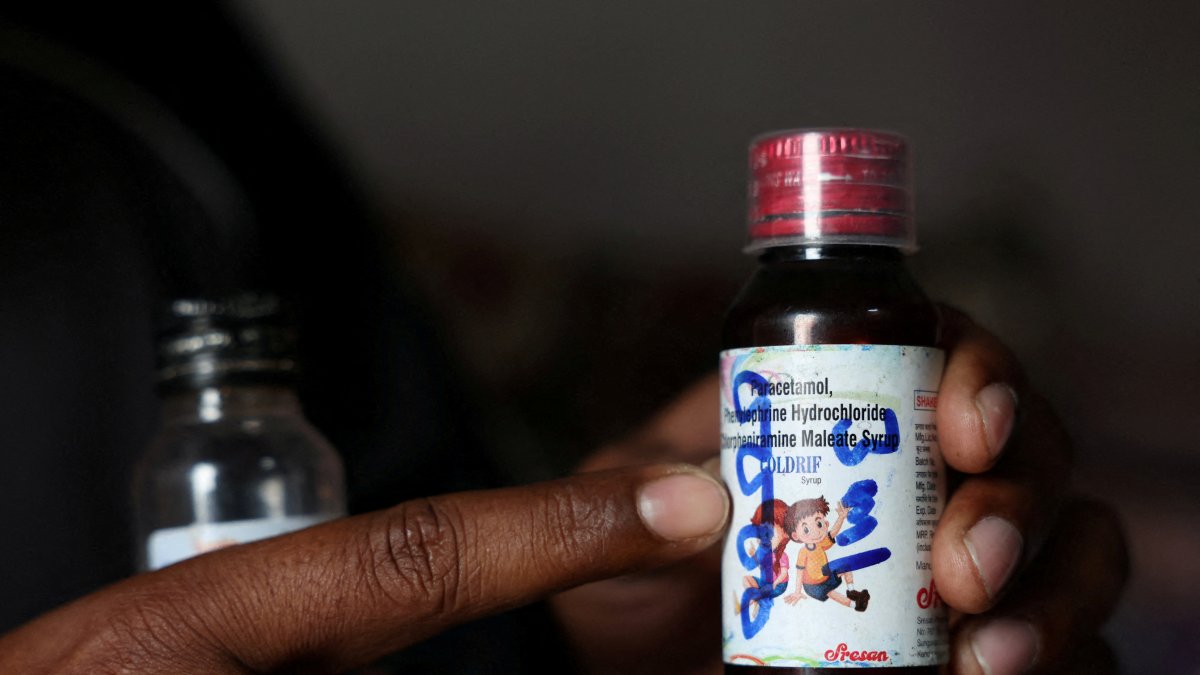
Indian authorities are probing whether a contaminated pharmaceutical solvent slipped through a chain of unlicensed suppliers and into a batch of cough syrup that killed at least 24 children, a case that has reignited fears over safety standards in one of the world’s largest drug-manufacturing hubs.
Three senior health and drug-safety officials in Tamil Nadu told Reuters they suspect the solvent used in the Coldrif cough syrup – propylene glycol, or PG – was tainted with the industrial toxin diethylene glycol (DEG) before it reached the manufacturer, Sresan Pharmaceutical.
Investigators say the contamination likely occurred around the time the PG was supplied to Sresan on
Continue Reading on Daily Sabah
This preview shows approximately 15% of the article. Read the full story on the publisher's website to support quality journalism.